
Prof.dr Ing. J.F.J van den Brand, Sep. 2006 – 8






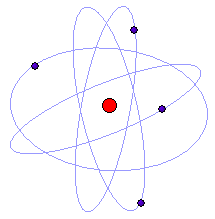

| However it was
in 1912 that the New Zealander Ernest Rutherford gave us our modern view of
the atom when he showed that atoms have a positive nucleus surrounded at a
relatively large distance by the electrons, a picture that everyone today
recognizes as the symbol for the atom. |
|
| The photograph
shows Rutherford in his lab. The sign says ‘Talk softly please’ supposedly
put there because the detectors were sensitive to noise. However the more
likely explanation is that it was aimed at Rutherford by his colleagues,
since he was renowned for his booming voice. |
|
| Now it became
clear what differentiates the elements - the number of electrons and the
charge on the nucleus - for example hydrogen has one electron, helium has
two, carbon six, lead eighty-two etc. |
|
| However the
story doesn’t stop there. |